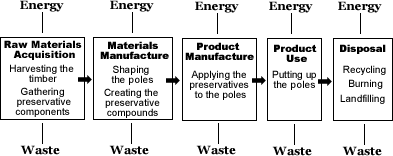
UTILITY POLES: LEARN ABOUT THE LIFECYCLE OF WOOD UTILITY POLES
Utility poles are a common feature of our everyday landscape. They support the wires that bring electricity from the power company to our homes and enable our growing network of telephones, televisions, and computers. Though wires are often buried underground in new developments, there are still roughly 120 million utility poles in service in the United States (AISI, 2005). Of these millions of poles, approximately 40% are owned by investor held utilities, 27% are owned by rural electricity associations, 28% by telephone companies, and 6% are owned by railroad companies (AWPA, 2005).
In order to conduct a life cycle analysis, we must first understand the inputs and outputs of the process from the "birth" to the "death" of the pole. The first step is to inventory the materials that go into a utility pole. Most utility poles are made of wood pressure-treated with some type of preservative to keep away woodpeckers, insects, fungi, and fires. Many different types of trees can be used to make utility poles, including Douglas fir, Jack Pine, Lodgepole Pine, and Pacific Silver Fir. Western Red Cedar is also popular for its natural insecticidal properties and durability, though its higher price deters many utility companies. The majority of utility poles are made from Southern Yellow Pine treated with a preservative called "Chromated Copper Arsenate" or simply CCA, made of a combination of chromium, copper, and arsenic. Other popular preservatives include Creosote and Pentachlorophenol (Penta).
Raw Material Acquisition & Materials Manufacture
Wood
Timber harvesting generally consists of five components: felling; cutting trees to standard lengths and removing un-usable limbs and tops; moving trees from the woods to a landing area - sometimes called skidding or yarding; loading the poles on trucks; and hauling the poles to the processing point (CORRIM, 2004). After de-barking the poles are shaped and cut into the specific dimensions. Utility poles can range anywhere from 20' to 125' depending on their eventual use but, on average, most utility poles are around 40 feet tall (Green and Hernandez, 1998). To air dry the material in preparation for the preservative treatment requires from 9 months to a year in storage in the yard. Using kilns to shorten the drying time is effective but is expensive for the timber mill. In terms of energy use, the inputs are greatest for kiln-drying because heat is often generated using non-renewable natural resources. In addition, the waste products from harvesting are often used in chipboard, paper pulp, etc., though un-usable cuttings may be left behind in the forest to decay.
According to the Western Wood Preservers Association, 'most poles come from maturing second growth stands which provide the straight, tall, sound and reasonably tapered timber needed' (Hayward, 1999). Tighter restrictions governing the number of trees, type of forest, and endangered species habitats that can be logged decreased the percentage of trees taken from federal lands over the last 50 years. However, privately held lands that were harvested in the late 1800's to the mid twentieth century now make up most of the second growth stands that produce the prime pole materials ( Hayward , 1999). Logging the trees not only uses energy, often generated through the burning of fossil fuels, but also produces greenhouse gases, such as carbon dioxide, as waste products. However, compared to alternative materials such as concrete and steel, wood materials have relatively small energy requirements. And the remaining forests continue to offset some of the released carbon by acting as 'carbon sinks' (Sedjo, 2001). Even when cut, wooden utility poles continue to embody a large amount of carbon (Sedjo, 2001).
Preservatives
There are many different types of chemicals that are used to preserve utility poles from insects, rot, fungi, and fires, but in this essay we focus on the most common types currently found in the United States : Creosote, CCA, and Penta. Coal-tar creosote has been used as a wood preservative in the U.S. for over 100 years. The creosote used in wood preservation is produced by the high temperature carbonization of coal and consists principally of aromatic hydrocarbons plus some tar acids and bases (AWPA, 2005). CCA, another type of preservative, is made up of the oxides or salts of copper, chromium, and arsenic. The arsenic and copper are toxic to insects and fungi that prey on wood, while chromium is used to bond the two elements to the wood's cellular components (Chirenje et al, 2003; HowStuffWorks.com). Up until 1984 Penta (C6HCl5O) was one of the most heavily used all-purpose pesticides in the U.S. Penta is produced using aluminum chloride or ferric chloride as catalysts for the chlorination of phenols (ATSDR, 2001).
Waste from Preservative Manufacture
The main wastes generated by preservative manufacturing are chemical compounds formed during the manufacturing process and excess amounts of the preservatives themselves. Contact with some of these chemicals can be harmful to organisms at certain thresholds. The wastes may be expelled into the atmosphere, carried away in waste water, or generate sludge. For example, during the manufacturing process of Penta, waste contaminants such as polychlorinated phenols, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans, are formed and may be released in to the environment outside the plant (ATSDR, 2001). Traces of the preservative have also been detected in rainwater, fish, crops, and the human body (HazDat, 2001). In the creation of creosote, sludge is fixed, solidified, and covered with clay to limit further contamination (ATSDR, 2002). Out of concern for the effects of these chemicals building up in the environment, preservatives such as Penta and Creosote have been banned in some European countries and restricted in the U.S. Currently the U.S. EPA limits the use of Penta, Creosote, and CCA to utility poles, pilings, and the like (ATSDR, 2001) in order to lessen public contact with the treated wood.
Product Manufacture
Once the pole has been shaped, and the preservatives created, the next step is combining the two products in a process called 'pressure-treating.' In this procedure the wood is soaked in a liquid preservative then placed in a pressure chamber which forces the chemical into the wood. The treatment process is typically controlled by a computer system. In the United States , the standard amount of preservative required within a utility pole is 0.40 pounds of chemical per cubic foot of wood (HowStuffWorks.com).
Cox Lumber describes their process: 'The lumber, timbers, and plywood to be treated are loaded onto small rail or tram cars. Using a vehicle such as a forklift, the trams are pushed into a large horizontal treating cylinder. The cylinder door is sealed, and a vacuum is applied to remove most of the air from the cylinder and the wood cells. Preservative solution is then pumped into the cylinder and the pressure raised to about 150 pounds per square inch, forcing the preservative into the wood. At the end of the process, excess treating solution is pumped out of the cylinder and back to a storage tank for later reuse. The cylinder door is opened and the trams are pulled out. The wood is wet at that time, so it is kept on a concrete pad. Any drips trickle into a containment area from which they can be either disposed of or reused' (Cox, 2005). Typically waste water, preservative drippings, and spent formulations from the wood preserving process contain chemicals that must be monitored to limit the release of these potentially harmful waste products into the atmosphere and waterways. After the wood has been pressure-treated it is then often thoroughly 'baked' in a kiln in order to further bond the chemicals to the wood and remove as much moisture as possible before the pole is sold to a utility company. Though it requires extra energy up-front, kiln drying recovers the environmental cost, by reducing the leaching rate of the chemicals applied to the pole. Extending the lifespan of the pole means ultimately that less energy will be used and less waste will be expelled in order to produce the necessary number of utility poles.
PRODUCT USE: In-Service Poles
The next step in a lifecycle analysis is understanding the energy used and waste produced during the product's use. Trucking is the most common means of transportation used to get utility poles to their first "job.'' From the processing plant, poles are either transported on flatbed trucks or self-unloading trucks with attached cranes. Rail transport is normally only used when distances from origin to the delivery site are long. The poles are then transported from the pole yards to the utility company that will put it up, and, from there, the utility company trucks the pole to its final destination. At the final destination, line beds and/or cranes are used to lift and set the poles into the ground. Each segment of the transport process requires fuel to operate the trucks and expels wastes, such as carbon dioxide, carbon monoxide, and nitrogen oxides from the truck's tailpipe. Once the pole is in place, in terms of energy consumption there is not much of a difference between a steel utility pole and a wooden utility pole. Most of the disparity has to do with greater energy inputs required for more frequent maintenance of wooden poles over substitute materials.
The waste produced by in-service poles is typically generated by the re-application and leaching of wood preservatives. With proper maintenance, the average lifetime of a wood utility pole is typically 30 to 40 years (Beyond Pesticides, 2005; AISI, 2005; Western Wood Preservers Institute, 1996), but as poles age, the effects of initial preservative treatments wear off and the preservatives must be re-applied (Wolfe et al, 2001). The majority of wood poles in service today has received, or is scheduled to receive, these repeat applications of preservative (WWPI, 2005).
During the time a utility pole is in use, water acts as a medium for preservatives leaching from the wood into soils and groundwater. Leaching rates vary by both type of wood and chemical applied as well as by the standard of application. Researchers estimate that between 30-80% of the Penta applied to wood is released within the first year, but CCA-treated wood, on the other hand, is more resistant to leaching than Penta-treated poles (Bunce and Nakai, 1989; Zagury, et al., 2003). Leaching rates also are effected by the amount of preservative initially absorbed into the wood; the pH of rainfall and soil near the in-service pole; as well as the type of soil the pole is rooted in. While the preservative chemicals do leach from the wood, generally, levels are highest immediately adjacent to the poles, and decrease to within normal levels within about a foot of the pole (Zagury, et al., 2003).
DISPOSAL
The final step in the lifecycle of a utility pole is disposal at the end of its in-service life. According to the North Pacific Group, "There are approximately 150 million wood poles in service throughout the United States with an additional six million new poles added annually. Approximately three percent of treated wood poles are retired from service each year" (North Pacific Group, 2005). At the end of its lifespan, a wooden utility pole is typically disposed of in one of three ways: deposited in a landfill, incinerated, or re-cycled for other uses. In each option, the release of the chemical preservatives into the environment is a concern. Most utility poles are currently disposed of in landfills. Though the preservatives are known to leach into the soil, most preservative-treated wood is not considered hazardous waste at the federal level, due to a loophole in the definition.
Burning releases the carbon sequestered in the wood, as well as the remaining chemical preservatives into the atmosphere. Burning CCA-treated wood, for example, releases copper, chromium, and arsenic into the atmosphere. The amount released is dependent on the temperature at which the wood is burned - "Belgian researchers have shown arsenic losses of about 22% from CCA-treated wood burning at low temperatures but losses of up to 77% at high temperatures (Helsen and Van Den Bulck). In the study, copper and chromium losses did not follow the same pattern, but there was evidence that the length of burning time affects the amount of each element released into the air.
Another option for out-of-service utility poles is to re-use the wood for other purposes. Poles currently coming out of service were most likely treated with CCA, Penta, or Creosote. All three have now been taken off the consumer market due to their toxicity, but regulations are variably enforced. Treated poles are sometimes recycled as mulch or other landscaping material, but a study conducted by Townsend and Solo-Gabriele (2001) revealed that two of three retail samples of colored mulch failed to meet regulatory guidelines for arsenic," (Clausen). Another option is to shave off the outer part of the wood that absorbed the preservative and to re-mill the preservative-free wood cores as decking or other lumber products. Poles are also sometimes chipped for use in composite materials, though preservatives make the chips difficult to bond together. Processing techniques must also meet certain air quality standards and control human exposure to untreated wood dust containing preservative compounds.
Due to current regulations, recycling wood poles is currently not as popular as disposing of them in a landfill. However, if preservatives such as Penta, Creosote, and CCA are classified as hazardous materials, tipping fees would greatly increase and modifications to the dumping grounds would have to be made; most would be required to install liners to limit chemical leaching into the soil and water supplies on site. The extra costs could make dumping prohibitively expensive and recycling an appealingly economical option for utility companies. In the meantime, the stricter regulations passed in the last ten years for Penta, Creosote, and CCA, are supporting the development of newer, more environmentally friendly preservatives.
Chemicals such as copper naphthenate (CuNap) and Alkaline Copper Quaternary (ACQ) are just two of the more "environmentally friendly" wood preservatives that have gotten approval since the other preservatives began to be more tightly regulated. ACQ uses a quaternary solution (a surfactant/cleaner) instead of arsenic as an insecticide. CuNap is also arsenic and chromium free, and is not restricted for use like Penta, Creosote, and CCA. Another option is to use more naturally decay-resistant species such as chestnut or tropical hardwoods. These species require fewer preservative applications over their lifespan than the cheaper, more commonly used pine woods - potentially decreasing both the number of poles required over time and the amount of energy needed and waste produced by using wood for utility poles (EPRI, 2004).
Updated by Nicole Barone Callahan Source